A groundbreaking development in the fight against cancer has emerged as Russian scientists announce that a revolutionary cancer vaccine Enteromix is ready for clinical use after successful trials. This positive milestone in medical science could reshape how cancer is treated, offering new hope for millions worldwide. The vaccine, based on advanced mRNA technology—the same used in some highly effective Covid-19 vaccines—has shown remarkable results in shrinking or slowing tumor growth by up to 80%.
What Makes the Enteromix Cancer Vaccine a Breakthrough?
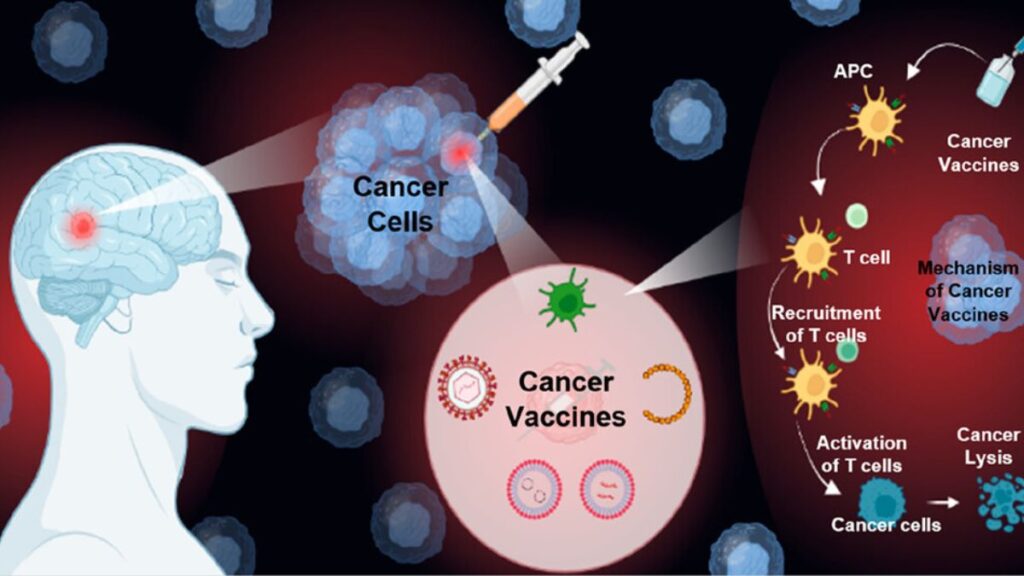
The cancer vaccine, Enteromix, is developed by Russia’s Federal Medical and Biological Agency (FMBA) under the leadership of Veronika Skvortsova. Unlike traditional therapies, this vaccine doesn’t rely on weakened viruses or invasive methods. Instead, it uses messenger RNA (mRNA) to instruct the body’s own cells to produce specific proteins that activate an immune response against cancer cells.
This innovative approach is designed to help the immune system recognize and attack cancer more efficiently, providing a safer and potentially more effective treatment option.
Proven Results from Preclinical Trials
Enteromix has undergone rigorous research, including three years of mandatory preclinical trials. The results have been very promising:
- Safe with repeated doses: Trials confirmed that the vaccine did not cause harmful side effects, even after multiple administrations.
- High effectiveness: Tumor growth was slowed or reduced by 60% to 80%, depending on the type of cancer.
- Broad potential: Early results suggest the vaccine could be effective against different cancers, with colorectal cancer being the initial focus.
Such results indicate a massive step forward in cancer immunotherapy, offering hope to patients who may not respond well to conventional treatments like chemotherapy or radiation.
Focus Areas: Colorectal Cancer, Glioblastoma, and Melanoma
The FMBA revealed that the first clinical application of Enteromix will target colorectal cancer, one of the leading causes of cancer-related deaths worldwide. Beyond this, researchers are also working on expanding the vaccine’s application to treat:
- Glioblastoma – a highly aggressive brain cancer with limited treatment options.
- Melanoma – a dangerous form of skin cancer, including ocular melanoma, which specifically affects the eye.
If successful, these applications could revolutionize cancer treatment, providing targeted and less harmful alternatives compared to existing methods.
Announced at the 10th Eastern Economic Forum
The official announcement of Enteromix was made during the 10th Eastern Economic Forum in Vladivostok, where more than 8,400 participants from over 75 countries gathered. This global platform highlights the vaccine’s potential impact, not only for Russia but also for international medical research and cancer treatment strategies.
Why This Breakthrough Matters Globally
Cancer continues to be one of the leading causes of death worldwide, with millions of new cases diagnosed each year. Traditional treatments often come with severe side effects and are not always effective. The Enteromix cancer vaccine represents a beacon of hope:
- Personalized treatment: Tailored to trigger the body’s own defense system.
- Minimally invasive: Reducing dependency on chemotherapy and radiation.
- Cost-effective potential: In the long term, vaccines like Enteromix could reduce healthcare costs significantly.
With its strong trial results and advanced technology, this breakthrough could redefine how the world approaches cancer therapy.
Enteromix Cancer Vaccine: Key Information
| Category | Details |
|---|---|
| Vaccine Name | Enteromix Cancer Vaccine |
| Developed By | Federal Medical and Biological Agency (FMBA), Russia |
| Leadership | Veronika Skvortsova, FMBA Head |
| Technology Used | Advanced mRNA Technology (Similar to COVID-19 vaccines) |
| Trial Duration | 3 Years of Mandatory Preclinical Trials |
| Success Rate | 60%–80% Reduction in Tumor Growth, depending on cancer type |
| Safety | Proven Safe with Multiple Doses, No Harmful Side Effects |
| First Target Cancer | Colorectal Cancer |
| Other Cancers in Focus | Glioblastoma (Brain Cancer), Melanoma (Including Ocular Melanoma) |
| Clinical Use Status | Reported Ready for Clinical Use |
| Announcement Platform | 10th Eastern Economic Forum, Vladivostok |
| Global Participation | 8,400+ Participants from 75+ Countries Attended |
| Key Benefits | Personalized Treatment, Minimally Invasive, Potentially Cost-Effective |
| Comparison with Chemo/Radiation | Targets Cancer Cells Precisely, Less Harm to Healthy Cells |
| Future Potential | Global Collaboration, Broader Clinical Trials, Possible Worldwide Use |
Final Thoughts
The cancer vaccine Enteromix ready for use after successful trials is more than just medical news—it’s a positive turning point in global healthcare. With its mRNA foundation, high effectiveness, and focus on deadly cancers like colorectal cancer, glioblastoma, and melanoma, Enteromix could save countless lives in the years to come.
As clinical applications begin, the world will be watching closely. If the vaccine delivers the same results in real-world usage as it did in trials, this could mark the dawn of a new era in cancer treatment, offering hope where it was once scarce.
Note: All information and images used in this content are sourced from Google. They are used here for informational and illustrative purposes only.
Frequently Asked Questions (FAQ)
Q1. What is the Enteromix cancer vaccine?
The Enteromix cancer vaccine is a new mRNA-based therapy developed by Russian scientists to help the body’s immune system recognize and fight cancer cells.
Q2. Who developed the Enteromix vaccine?
The vaccine was developed by the Federal Medical and Biological Agency (FMBA) of Russia, under the leadership of Veronika Skvortsova.
Q3. How does the Enteromix cancer vaccine work?
Enteromix uses mRNA technology to instruct the body’s cells to produce proteins that trigger an immune response against cancer cells, helping the immune system target and destroy them.
Q4. What cancers will Enteromix target first?
The vaccine will first be used for colorectal cancer. Research is also advancing for glioblastoma (a fast-growing brain cancer) and melanoma, including ocular melanoma (affecting the eye).
Q5. How effective is the Enteromix vaccine?
Preclinical trials showed that the vaccine was safe even with repeated doses. In some cases, tumors shrank or grew more slowly by 60% to 80%, depending on the cancer type.
Q6. Is the Enteromix vaccine safe?
Yes, preclinical trials confirmed that the vaccine is safe and does not cause harmful side effects, even with multiple doses.
Q7. Where was the Enteromix vaccine announced?
The official announcement was made during the 10th Eastern Economic Forum in Vladivostok, which was attended by over 8,400 participants from more than 75 countries.
Q8. When will the Enteromix cancer vaccine be available for patients?
The vaccine is reported to be ready for clinical use. However, widespread availability will depend on regulatory approvals and further clinical applications.
Q9. How is this vaccine different from chemotherapy or radiation therapy?
Unlike chemotherapy or radiation, which can damage healthy cells along with cancer cells, Enteromix is designed to train the immune system to specifically target cancer cells, making it a potentially safer and more precise treatment.
Q10. Could Enteromix be used worldwide?
Currently, the vaccine is being developed and trialed in Russia. Its global availability will depend on international trials, regulatory approvals, and collaborations with healthcare organizations.







Leave a Reply